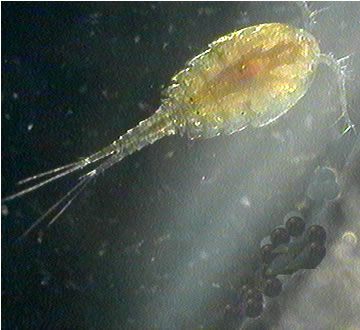
Ecology and Behavior |
|||||||||||||||
Characteristics |
|||||||||||||||
|
|||||||||||||||
Similar Species |
|||||||||||||||
| Acanthocyclops robustus is often confused with Acanthocyclops vernalis; Although these were once classified
as the same species. Acanthocyclops robustus is now considered separate because of variations in habitat and behavior (6). Mating errors may occur between A. robustus and Mesocyclops leukarti as similar sized individuals of one species mistakenly attempt to mate with the other species (4). |
|||||||||||||||
Geographic Distribution |
|||||||||||||||
| Reported locations include (but are not limited to) Central Europe (4)(10); the Netherlands (2); Germany (5); Poland (8); Oklahoma, North American Great lakes and New England (USA). | |||||||||||||||
Habitats |
|||||||||||||||
|
In highly productive lakes, A. robustus is more successful in the littoral zone than the pelagic zone (3)(4). A. robustus prefers the mud layer of the littoral zone during resting stages (4). A. robustus tolerates brackish waters of estuaries (2). |
|||||||||||||||
Food and Feeding Behavior |
|||||||||||||||
| Acanthocyclops robustus is predaceous and as adults they eat small-bodied cladocerans such as Daphnia magna, D. hyaline, D. pulicaria and D. cucullata (7). A. robustus selects on the basis of prey body shape, external cover, and mode of swimming rather than body size (7). Adult cladoceran species such as D. magna and D. pulicaria (> 7 days old) can coexist with A. robustus because of their large size, but cladoceran neonates (1 day old) and juveniles (3-5 days) may be heavily preyed upon (7). The first copepodite stages of A. robustus may invade brood pouches to feed on embryos and ovary material, particularly Daphnia magna, D. pulex, and D. pulicaria (5)(7)(10). A. robustus copepodites may eat one or two eggs in a 2-minute visit and in one case four eggs were devoured in 12-minutes (10). Changes in prey density (typically Daphnia spp.) alter A. robustus behavior. At high densities of prey, A. robustus swims in tight circles or "horizontal loops" in the horizontal plane of the water column, keeping close proximity to higher densities of Daphnia (9)(11). In the laboratory, A. robustus were observed eating live carp larvae (8). |
|||||||||||||||
Reproductive Habits |
|||||||||||||||
| A. robustus copepodites emerge from diapause in spring (4). Reproduction is highest in the late summer and fall (3)(4). A. robustus produces exceptionally large egg clutches with the highest in the spring (3)(4). Mean number of eggs per egg-bearing female varies between 40.4 and 74.5 (4). The length of egg sac is approximately 0.36 mm or 1/3 the body size of the adult (4). | |||||||||||||||
Predation on Acanthocyclops |
|||||||||||||||
| A. robustus populations may contain a high ratio of males to females (28:1) due to fish predation (3)(4). Although this species is relatively small, the females are larger than the males (4). The large egg sacs makes it difficult for the small female A. robustus to swim rapidly and escape fish predation; their success in the pelagic zone of eutrophic lakes is reduced when planktivorous fish are abundant (3). | |||||||||||||||
Competition |
|||||||||||||||
| Mesocyclops leuckarti may compete with A. robustus because both are similar in size, inhabit the same water layers, and have similar seasonal occurrence (1)(4). Although A. robustus typically consumes more prey than M. leuckarti (4), it produces larger clutch sizes and has more rapid embryonic development than M. leuckarti; larger clutch sizes make A. robustus females more vulnerable to visual predators than M. leuckarti (4). | |||||||||||||||
Migration |
|||||||||||||||
| Acanthocyclops robustus display diel vertical migratory patterns (DVM). Downward migration begins at sunrise and upward migration at sunset. A. robustus is more abundant near the bottom of the water column during the day and more evenly distributed during the evening (9). DVM aids in avoiding fish predators such as perch and pikeperch (9). Adults, copepodites and nauplii may have different vertical distributions in the same lake (9). |
|||||||||||||||
Seasonal Variations |
|||||||||||||||
| A. robustus population reaches its maximum in spring to early summer between the months of June and September (2)(4). Reproduction is highest in summer in the littoral zone (2)(3)(4). A. robustus is smaller in size during summer and larger in spring and autumn (4). | |||||||||||||||
References |
|||||||||||||||
|
(1) Vijverberg, J and A. F. Richter. 1982. Population dynamics and production of Acanthocyclops robustus (Sars) and Mesocyclops leuckarti (Claus) in Tjeukemeer. Hydrobiologia. 95: 261-274. (2) LIONARD, M., F. AZÉMAR, S. BOULÊTREAU, K. MUYLAERT, M. TACKX, AND W. VYVERMAN. 2005. Grazing by meso- and microzooplankton on phytoplankton in the upper reaches of the Schelde estuary (Belgium/The Netherlands). Estuar. Coast. and Shelf Sci. 64: 764-774. (3) MAIER, G. 1998. Differential success of cyclopoid copepods in the pelagic zone of eutrophic lakes. J. Marine Syst. 15: 135-138. (4) MAIER, G. 1990. Coexistence of the predatory cyclopoids Acanthocyclops robustus (Sars) and Mesocyclops leuckarti (Claus) in a small eutrophic lake. Hydrobiologia. 198: 185-203. (5) GLIWICZ, Z. M. AND W. . 1994. Clutch size variability in Daphnia : Body size related effects of egg predation by cyclopoid copepods. Limnol. Oceanogr. 39: 479-485. (6) PURASJOKI, K. AND H. VILJAMAA. 1984. Acanthocyclops robustus (Copepoda, Cyclopoida) in plankton of the Helsinki sea area, and a morphological comparison between A. robustus and A. vernalis: Deep-Sea Res. Pt. B. Oceanograph. Lit. Rev. 250: 33-44. (7) GLIWICZ, Z. M. AND G. UMANA. 1994. Cladoceran body size and vulnerability to copepod predation. Limnol. Oceanogr. 39: 419-424. (8) PIASECKI, W. G. 2000. Attacks of cyclopoid Acanthocyclops robustus (Sars) on newly hatched Cyprinids. Electronic Journal of Polish Agricultural Universities, Fisheries. 3: 1-14. (9) ROCHE, K. 1990. Spatial overlap of a predatory copepod, Acanthocyclops robustus, and its prey in a shallow eutrophic lake. Hydrobiologia. 198: 163-183. (10) GLIWICZ, Z. M. AND H. STIBOR. 1993. Egg predation by copepods in Daphnia brood cavities. Oecologia. 95: 295-298. (11) WILLIAMSON, C. E. 1981. Foraging behavior of a freshwater copepod: frequency changes in looping |
|||||||||||||||
| Additional Photographs | |||||||||||||||
|
|||||||||||||||
| Quicktime Movies | |||||||||||||||
Cyclopoid1.mov - Cyclopoid swimming patten (682 KB) |