| Ecology and Behavior | |||||
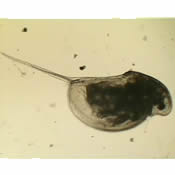
| Characteristics | |||||
|
|||||
| Similar Species | |||||
| D. middendorffiana have similar morphological features with D. pulex, resulting in uncertainty as to the relationship between the two species (3). | |||||
| Geographic Distribution | |||||
D. middendorffiana distributions are circumpolar extending southward into the high elevations of the Rocky Mountains and Pacific Coastal mountain ranges in North America and the Alps in Europe (3). This species has been found in the highly productive, Los Juncos Pond in Argentina (4); oligotrophic Toolik Lake and tundra ponds in Alaska and waterbodies in Sweden (5)(6)(7); Rocky Mountain ponds (1) and oligotrophic, alpine Pipit Lake, Banff National Park, Alberta, Canada (3). |
|||||
| Reported Habitats | |||||
| Found at extreme high latitudes and enriched polar lakes and ponds (2); often in semi-permanent, shallow, fishless bodies of water (4). D. middendorffiana is eurythermal but has a thermal range for respiration, approximately 5 degrees C lower than that for temperate zone Daphnia spp. (6). |
|||||
| Food and Feeding Behavior | |||||
| D. middendorffiana can occur in Arctic lakes and ponds with low phytoplankton concentrations(3). | |||||
| Predation on Daphnia | |||||
|
|||||
| Competition | |||||
| In Arctic ponds where H. septentrionalis is not present, D. pulex or Bosmina will be the likely dominant species (7). H. septentrionalis is 40 times more likely to capture D. pulex than D. middendorffiana. A possible explanation for resistance to predation is the strong carapace of D. middendorffiana protects the zooplankton from predators that bite parts off its prey rather than engulfing prey intact (7).
In a study by Dodson (1984), the carapace strength of D. middendorffiana was approximately 3.1 times stronger than the carapace of comparably sized D. pulex (1). D. middendorffiana has a lower reproductive rate than D. pulex. This may be a result of energy expenditure for the development of a stronger carapace and larger body size to avoid capture by H. septentrionalis (2). | |||||
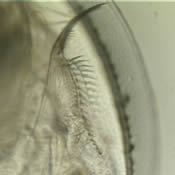
| Additional Pictures | |||||
|
|||||
| References | |||||
(1) Dodson, S.I. 1984. Predation of Heterocope septentrionalis on Two Species of Daphnia: Morphological Defenses and Their Cost. Ecology. 65: 1249-1257. (2) Haney, J.F. and C. Buchanan . 1987. Distribution and biogeography of Daphnia in the arctic. In Peter, R.H. and DeBernardi, R. (eds), Daphnia . Mem. Ist. Ital. Idrobiol. 45: 77-105. (3) Wilhelm F.M., A.K. Hardie, A.S. McNaught and S.L. Clare. 1998. Large Suprabenthic Daphnia middendorffiana from an Alpine Lake in the Canadian Rocky Mountains. Canadian Field-Naturalist 112: 419-424. (4) Vega M.P. and A. Clause. (2000). Morphometry and Cyclomorphosis in Daphnia middendorffiana from a Fishless Pond of the Southern Andes. Journal of Freshwater Ecology. 15 (3): 329-338. Oikos Publishers. (5) Peterson, B.J., J.E. Hobbie, and J.F. Haney. (1978). Daphnia Grazing on Natural Bacteria. Limnol. and Oceanogr. 23 (5): 1039-1044. (6) Yurista, P.M. (1999) Temperature-dependent energy budget of an Arctic Cladoceran, (7) Luecke, C. and W.J. O'Brien. (1983). The Effect of Heterocope Predation on Zooplankton Communities in Arctic Ponds. Limnol.and Oceanogr. 28(2): 367-377. American Society of Limnology and Oceanography. (8) Dodson, S.I. and D.L. Egger. (1980). Selective feeding of Red Phalaropes on zooplankton of arctic ponds. Ecology 61: 755-763. (9) Vega, M.P. and A. Clausse. (1998) Hydrodynamic Characteristics of Daphnia middendorffiana. International Review of Hydrobiology 83(4): 267-277. |
|||||