|
|
||||
|
General Biology: With each cell division, one daughter cell is slightly smaller than the parent cell, while the other daughter cell remains the same size. This is the result of the hypotheca valve lying within the epitheca valve, and upon division the hypotheca becomes the epitheca of the daughter cell, thus repeating the reduction in frustule size on each new division. Induction of gametes occurs when, after diminishing in size after several asexual divisions, a diatom cell reaches a minimum size, usually 45 to 55% (but in some species 30 to 75%) of their maximum size. (Edlund and Stoermer 1997). Reduction division (meiosis) was first demonstrated by Klebahn (1896) in Rhopalodia, and later by Karsten (1912) in Surirella. The diplontic life cycle of all diatoms has been accepted since that time (van den Hoek et al. 1995), and it is almost unique among the algae, the only other case being Vaucheria in class Tribophyceae (Mann 1993) -- they are diploid except for meiotic production of gametes (gametic meiosis). Sexual reproduction of diatoms has been reviewed (Ibid). Sexual reproduction is an obligate stage in the life cycle of most species. The life cycle can take months to years, while gametogenesis and fertilization usually take just hours to complete. In some cases plasmogamy occurs without karyogamy, resulting in a prolonged period of the dicaryon stage. Then the zygote (or dicaryon) matures into an auxospore that may take up to a month to develop. The auxospore often has little resemblance to vegetative cells, into which it germinates (Chepurnov et al. 2004). If the sexual stage fails and is bypassed by continued size depletion the small cells reach the end of their cycle and die. With the exception of zoogametes in a few centric species, diatoms lack flagella. Some pennate diatoms (monoraphes and biraphes) have a rapid “gliding motility” on solid surfaces, excreting a mucilaginous propellent from a raphe (elongate “slit” in the raphe, exposing the cell membrane to the solid surface). A few centric diatoms have a “shuffling motility” on the same basis, involving the labiates process (rimoportula) rather than a raphe, but much slower than the pennates (Medlin et al. 1986). Because their silica frustules diatoms are more dense than most photosynthetic plankton and most are meroplankton requiring water currents to remain in suspension. A few, especially the elongate centric genus Rhizosolenia, are euplanktonic, decreasing their specific gravity by accumulating less silica in their frustules and carrying lipid droplets (less dense than water). Diatoms, thought to have had flagella and lost them through time, are also grouped with other classes such as goldens and browns that have in common two different types of flagella in the motile stages, into the "stramenopiles" ("straw hairs") and/or "heterokonts" ("differing poles", i.e. flagella). Included are both photosynthetic and heterotrophic forms. They are also grouped into the ochrophytes ('ocher-colored plants', although they are protists). Date of origin of diatoms is unclear but undisputed fossils date to the Upper Cretaceous (100 - 65 million years ago), with some evidence of fossils from the Lower Crecaceous Period (143 - 100 mya). Likely they are monophyletic based on the universality of two valves and "girdle bands" (Round and Crawford 1981). It is also argued that the date of origin could have been much earlier, as long as 2 billion years ago (2 Ga) -- late Proterozoic through late Jurrasic, ~ 2000 - 143 mya (Ibid.) Biosilicification Biosilica structures have
increased strength because of their organic–inorganic microstructure, and
as a result diatom frustules are the strongest biological materials per unit
density known to science (Aiken et al. 2016). Brief History of Diatom Discovery: I am grateful to David Hollombe (California USA) for suggesting the following historical references: Improvement in resolving power of microscope lenses during the early 19th Century accelerated the pace of diatom taxonomy and classification based mainly on the complexity of their silica frustules. During the same time discovery of immense layers of diatomaceous clay in Europe and North America gave credence to the contemporaneous ‘Doctrine of Uniformitarianism’ (Lyell) and to the realization that the age of the earth was far greater than thought previously. All of this coincided with the conceptualization of the Origin of Species (Darwin 1859). Mining of clay at Pirkenhammer, Czech Republic (near Karlsbad) for production of porcelain (history of factory here) led to early diatom analysis by Kutzing, similar to mining in Massachusetts that spurred Bailey during the 1830s (Edgar 1981). Perhaps the classic early key to diatoms, at least freshwater forms, is Bacillariophyta (Diatomeae) (Hustedt 1930). An
excellent online
image-based key is being developed (since 2009) at the University of Colorado
at Boulder, by Sarah A. Spaulding, US Geological Survey Institute of Arctic
and Alpine Research. Age of Diatoms: Diatoms are relatively young among the photosynthetic eucaryotes. The earliest known fossil diatoms are from the Early Cretaceous, ~ 150 Ma. However, a continuous record of fossil diatoms is found in marine sediments dates from only ~65 Ma, during the demise of the dinosaurs, with increasing evolution of genera and species since that time.
Evolutionary time scale of diatom genus and species evolution (Kotrc 2013). Dispersal and Diversity: The
concept that microbes (both procaryotes and eucaryotes) are easily dispersed
and therefore ‘everything is everywhere’ (Baas-Becking 1934,
Finlay and Clarke 1999, Finlay 2002) , given that a
compatible habitat exists globally, has been challenged with the use of
diatom databases in both the north and south hemispheres of earth (Vyverman et al. 2007). The results indicate asymmetry between
north and south hemispheres, and suggest that mainly historical factors and
isolation of lakes (similar to prediction of the ‘island effect’
in ecology) play a major role in constraining dispersion –
approximately 70% role, while local environmental factors including
temperature and lake chemistry played a much lesser role (< 30%) in
determining the relative richness of genera and species of diatoms in various
lakes and lake regions. |
||||
|
Diatomite: |
||||
|
Marine deposits of diatom frustules, long
ago oxidized free of organic carbon, can be 90 - 95% pure silica with density
as low as 8% of water when dry (porous, with a large volume of air space), so
it floats unless saturated with water (Dolley 2000). Building blocks and
bricks have been made and used since ancient Greece because of their low
density and heat insulation properties. A well-known museum, built as a
cathedral, the Church of St. Sophia, later used as a mosque, in Istanbul
Turkey, 30 m wide at the base of the dome made from diatomite blocks to
reduce its weight:
Dome of St. Sophia, Istanbul, Turkey
from inside the museum, with radial symmetry, constructed during 532-537 AD
from blocks of diatomite.
View of Dome of St. Sophia, Istanbul, Turkey from outside the museum constructed in 523 AD from blocks of diatomite. Diatomite has been
valued as a mild abrasive since the time of ancient Greeks, and has been used
for polishing metals, in tooth paste, and as a
'filler' in cigars. Since the early 20th century AD diatomite has
been used as a filter to clarify beer (removing yeast cells) and as an
absorber and stabilizer of nitroglycerine forming dynamite (Dolley 2000).
|
||||
|
Nanofabrication
from complex frustules structure |
||||
|
Mimicking the complex structure of frustules that can grow two or three layers of porous silica, each layer different from the others and separated by vertical walls, shows promise in nanotechnology and is a focus of study, as in the Losic Group at the University of Adelaide:
The
above image from the research subgroup on NanoBiotechnology: Biomimetics and Nature Inspired
Nanotechnology is posted online. Diatom nanotechnology, including investigations
of how patterns within frustules develop, has commercial value, as discussed
by Drum
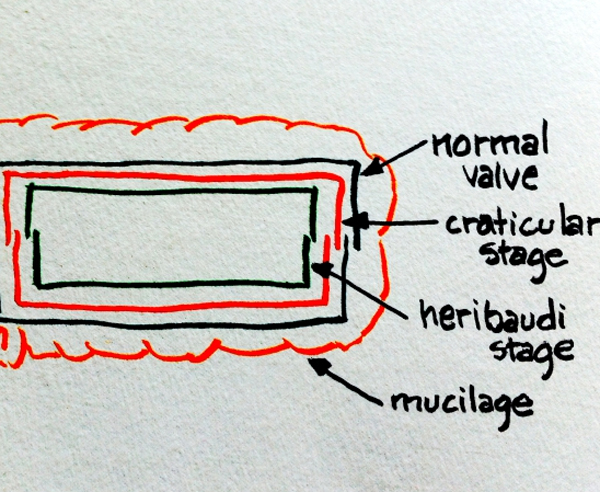
and Gordon (2003). Reproduction Sexual and asexual reproduction occur. Asexual reproduction is induced when frustules are diminished through successive divisions to a minimum size for a species. An ‘autospore’ that usually has a different morphology and is much enlarged, is produced that after a period of time transforms into a large vegetative cell that subsequently continues asexual mitotic division as a diploid (2N) cell. Diploidy in 'vegetative' cells is almost unique to diatoms among protists. A resting stage called the ‘craticular stage’ based on observations of Craticula are formed by a frustule producing two more layers of valves inside the 'normal' or outside frustules. A layer of mucilage is secreted to the outside and enables a prolonged resting period of up to eight years. Sexual reproduction includes meiosis and subsequent fusion of nuclei. Gametes in all pennate and most centric diatoms are nonflagellated, and migrate toward each other before fusing. Some species of centrics have an emergent flagellum.
Sketch
of craticular stage and heribaudi stage inside normal valve, the entire
package surrounded by a layer of mucilage. Credit to Sarah Spaulding,
posted online.
|
||||
|
More on morphology and morphogenesis |
||||
|
References: |
||||
|
Aitken, Z.H., A. Luo, S.N. Reynolds, C. Thaulow, and J.R. Greer 2016. Microstructure provides insights into evolutionary design and resilience of Coscinodiscus sp. frustule. Proceedings of the National Academy of . Science USA 113(8):2017-2012. Baas-Becking,
L.G.M. 1934. Geobiologie
of Inleiding tot de Milieukunde. Van Stockum and Zoon, The Hague, The
Netherlands. Chepurnov, V.A., D.G. Mann, K. Sabbe and W. Vyverman 2004. Experimentall studies on sexual reproduction in diatoms. International Review of Cytology 237:91-154. Dolley, Thomas P. (2000). Diatomite. US Geological Survey Minerals Yearbook.PDF-document Drum, R.W., and R. Gordon 2003. Star Trek replicators and diatom nanotechnology. Trends in Biotechnology 21(8): 325-327. Edgar, R.K. 1981. The origin of diatom biology in America. Occasional papers of he Farlow Herbarium, vol. 16, no. 43-44. [reset here]. Finley,
BJ., and K.J. Clarke
1999. Ubiquitous
dispersal of microbial species.
Nature 400:828. Hustedt,
F. 1930. Bacillariophyta (Diatomeae). In: Die Susswasser-Flora Mitteleuropas.
Heft 10. Jena. Verlag von Gustav Fischer. Kotrc, B. 2013. Evolution of Silica Biomineralizing Plankton. Doctoral dissertation, Harvard University. Lund, J.W.G., F.J. Mackereth, and C.H. Mortimer 1963. changes in depth and time of certain chemical and physical conditions and of the standing crops of Asterionella formosa Hass. in the North Basin of Windermere in 1947. Phil. Trans. R. Soc. Lond. Ser. B 731:255-290. Mann, D.G. Patterns of sexual reproduction in diatoms. Hydrobiologia 269-270:11-20. Round,
F.E., and R.M. Crawford 1981. The lines of evolution of the diatoms. I.
Origin. Proc. Royal Soc. London. Series B, Biological Sciences
211(1183):237-260. Vyverman, W., E. Verleyen, K. Sabbe, K. Vanyoutte, M. Steerken, D.A. Hodgson, D.G. Mann, S. Juggins, B. Ben de Vijver, V. Jones, R. Flower, D. Roberts, V.A. Chepurnov, C. Kilroy, P. Vanormelingen, and A. De Wever 2007. Historical processes constrain patterns in global diatom diversity. Ecology 88(8):1924-1931.
|
||||