|
Home / Greens / Unicells / Non-flagellated / Chlorella
|
||||
|
|
||||
|
Click
on images for larger format |
||||
Name derivation: |
||||
|
|
||||
Classification: |
||||
Chlorella M.Beijerinck
1890
Order Chlorellales; Family Chlorellaceae; 33 of 96 species descriptions are
currently accepted taxonomically (Guiry and Guiry).
Synonym: Muriella.
|
||||
Morphology: |
||||
|
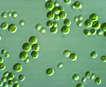
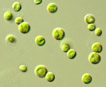
Green spherical to ellipsoidal unicells
with one chloroplast, often growing in groups. Asexual reproduction by forming 2- 8 autospores.
|
||||
Similar genera: |
||||
|
More than 100 taxa (probably
species descriptions) have been incorrectly designated Chlorella (Krienitz et al. 2014). |
||||
Calvin cycle: |
||||
Dark (light-independent)
reactions of PS use 9 ATP (fuel) and 6 NADPH (reducing power) molecules to
incorporate 3 CO2 molecules into a single precursor of sugars: glyceraldehyde 3-phosphate. Light reactions of PS produce the ATP
and NADPH. The cycle was
determined with Chlorella cultures (Calvin and Benson 1948).
|
||||
Biofuel potential: |
||||
High lipid content and growth
rate are a major considerations for selecting microalgal strains.
Of five Chlorella species, C. emersonii growing
in culture without a source of nitrogen (none was listed for the
‘low-N’ freshwater growth medium) had ~63% lipid content compared
to the control culture grown in Watanbe medium
(Watanabe 1960) with 1.25 g KNO3 L-1. Although the initial growth rate was
depressed (days 1-5) it then increased providing the highest yield after one
week. The protein content in
N-limited culture was decreased (Illman et al.
2000).
|
||||
Biogas
wastewater utilization:
|
||||
A model of biomethane
production using the cyanobactium Spirulina predicted a net energy ratio of 1.54
using an existing biogas plant (Wang et al. 2013), suggesting the value of
integrating microalgal production with biogas
waste.
A test of using industrial
wastewater is addition of biogas production wastewater containing 75-80% pure
glycerol with methanol, free fatty acids, a catalyst residue and other
impurities, added to BG11 medium minus nitrogen that supported mixotrophic growth.
Optimal growth was in the growth medium with 0.114 g L-1 N
and 2.7 g L-1 technical glycerol (Skorupskaite
et al. 2015).
|
||||
Dairy wastewater utilization: |
||||
Chlorella sp. developed 23% lipids in
ten days when grown on 80% dairy wastewater and 20% BG11 medium (Guruvaiah et al. 2012).
|
||||
Health
food competitor of Spirulina:
|
||||
Mass culture of Chlorella is
also targeting the health food industry with claims of body detoxification,
greater longevity and antioxidants (β-carotene).
|
||||
Habitat: |
||||
|
All aquatic habitats and wet subaerial
surfaces. |
||||
References: |
||||
|
Calvin, M. and A.A. Benson 1948. The path of carbon in photosynthesis. Science 107:476-480. Guiry, M.D. and Guiry, G.M. 2013. AlgaeBase. World-wide
electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on
18 April 2013. Guruvaiah, M., D. Shah and E. Shah 2014. Biomass and lipid accumulation of
microalgae grown on dairy wastewater as a possible feedstock for biodiesel
production. International Journal
of Science and Research 3(12):909-913. Illman, A.M., A.H. Scragg
and S.W. Shales 2000. Increase in Chlorella strains calorific values when grown in low nitrogen
medium. Enzyme and Microbial
Technology 27:631-635. Krienitz, L., V.A.R. Huss and C. Bock 2014. Chlorella: 125 years of the gre4en
survivalist. Trends in Plant
Science xx:1-3 (in press). Skorupskaite, V., V. Makareviciend
and D. Levisauskas 2015. Optimization of mixotrophic
cultivation of microalgae Chlorella
sp. for biofuel production using response surface
methodology. Algal Research
7:45-50. Watanabe, A. 1960. List of algal strains in the collection
at the Institute of Applied Microbiology, University of Tokyo. Journal of General Applied
Microbiology 6:283-292. Wang, X., E. Nordlander, E. Thorin and J. Yan 2013. Microalgal biomethane production integrated with an existing biogas
plant: A case study in
Sweden. Applied Energy
112:468-484. |
||||