|
|
|
|||
|
|
||||
|
Click on images for larger format |
||||
Name derivation: |
||||
|
From the Greek synecho, to hold together, and kokkos , a kernel, a grain, or berry |
||||
Classification: |
||||
|
Synechococcus Nägeli 1849; 39 of 80 species descriptions are currently accepted taxonomically (Guiry and Guiry 2013). Order Synechococcales; Family SynechococcaceaeSynonym: Cyanothece Komárek 1976.
|
||||
Morphology: |
||||
|
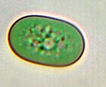
Range in size from 1 - 22 µm, cylindrical or rod-shaped unicell without mucilaginous sheath, cell longer than wide. Occurs singly or in loose colonies (Geitler 1932). |
||||
Similar genera: |
||||
|
There appears to be a morphological overlap between Synechococcus Nageli 1849 and Synechocystis Sauvageau 1892, the former described in more detail (Geitler 1932). It is likely the two genera are each polyphyletic given the number of lumping and splitting by a variety of systematists (Whitton 2011). |
||||
Habitat: |
||||
|
Important member of freshwater and marine plankton, where it is involved in calcium carbonate precipitation. Also found on the surface of algae and plants in hot springs and thermal pools. Also found associated with colonies of Coelosphaerium. Along with Prochlorococcus, Synechococcus contributes most of the photosynthetic productivity in tropical oceans (Ting et al. 2002). |
||||
|
Chromatic adaptation: |
||||
|
The red phycobilin pigment phycoerithrin increases ~x20 in light limited cells(< 30 quanta), and the ratio of phycoerithrin/phycocyanin increases to 14:1 (g/g)(Kana and Glibert 1987). Phycoerithrin also increases when cultures are grown in green light (Bryant, Penn State University online). |
||||
|
Use: |
||||
|
Synechococcus is a model in genetic studies since 1968. It is spontaneously transformable, able to integrate foreign DNA by homologus recombination. It was the first photosynthetic organism to have its entire genome sequenced (1996). Several studies on pigment synthesis and regulation and many others are summarized online by the laboratories of Vermaas and Roberson at Arizona State University. Synechococcus is considered to be an ideal organism for the production of organic products. It’s metabolic ‘network’ is summarized by Knoop et al. (2010). |
||||
Allelotoxins: |
||||
|
Synechococcus and its close neighbor Synechocystis produce a variety of antifungal and antibacterial toxins, but apparently not microcystin (Martins et al. 2007). |
||||
References: |
||||
|
Geitler, L. 1932. Cyanophyceae von Europa. In: Rabenhorst, L. Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. (1196 pp) Guiry, M.D. and G.M. Guiry 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 27 August 2013. Hodell D. A. and C. L. Schelske. 1998. Production, sedimentation, and isotopic composition of organic matter in Lake Ontario. Limnol. Oceanogr. 43: 200-214 Hodell D.A., C.L. Schelske, G. L. Fahnenstiel, and L. L. Robbins. 1998. Biologically induced calcite and its isotopic composition in Lake Ontario. Limnol. Oceanogr. 43: 187-199 Kana, T.M., and P.M. Glibert 1987. Effect of irradiances up to 2000 μE m-2 s-1 on marine Synechococcus WH7803 – I. Growth, pigmentation, and cell composition. Deep Sea Research Part A. Oceanographic Research Papers 34(4):479-485. Knoop, H., Y. Zilliges, W. Lockau, and R. Steuer 2010. The Metabolic Network of Synechocystis sp. PCC 6803: Systemic Properties of Autotrophic Growth. Plant Physiology 154:410-422. Martins, R.F., M.F. Ramos, L.Herfindal, J.A. Sousa, K. Skaerven, and V.M. Vasconcelos 2007. Antimicrobial and cytotoxic assessment of marine cyanobacteria – Synechocystis and Synechococcus. Marine Drugs 6(1):1-11. Nägeli, C. 1849. Gattungen einzelliger Algen, physiologisch und systematisch bearbeitet. Neue Denkschriften der Allg. Schweizerischen Gesellschaft für die Gesammten Naturwissenschaften 10(7): i-viii, 1-139, pls I-VIII. Thompson J. B., S. Schultze-Lam, T. J. Beveridge, and D. J. DesMarais. 1997. Whitening events: biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton. Limnol. Oceanogr. 42: 133-141. Ting, C.S., G. Rocap, J. King and S.W. Chisholm 2002. Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies. Trends in Microbiology 10(3):134-142. Whitton, B.A. Cyanobacteria. In: The Freshwater Algal Flora of the British Isles. Cambridge University Press. (878 pp). |
||||