|
Home / Cyanobacteria / Filaments / Pseudo-branches / Scytonema |
||||
|
|
|
|
||
|
|
|
|||
|
Click on images for larger format |
||||
Name derivation: |
||||
|
"Leathery thread" (from the Greek skytos, leather, and nema, thread) |
||||
Classification: |
||||
Scytonema C.Agardh ex Bornet and Flahault 1886; 109 of 209 species descriptions are currently accepted taxonomically (Guiry and Guiry 2013).Order Nostocales, Family ScytonemataceaeScytonema section Petalonema is considered synonymous with genus Petalonema (Geitler 1932). |
||||
Morphology: |
||||
|
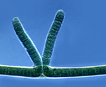
Trichomes of cylindrical cells inside firm sheaths. Filaments are often coiled and form entangled mats. Double false branching is frequent and results from the growth of the filament on either side of a formed necridium (dead cell) or of heterocysts. Reproduction by hormogonia (trichome fragments). |
||||
Similar genera: |
||||
|
Plectonema may exhibit similar double false branching, but its cells are discoid and it does not produce heterocysts. |
||||
Cryptobiosis: |
||||
Terrestrial strains of Scytonema in arid soils are subjected to salt stress during dehydration. Production of the two sugars sucrose and trehalose, both with the same chemical formula C12H22C11 but with different structure and function, increases and both remain high in concentration until subsequent rehydration (Page-Sharp et al. 1999). The same phenomenon occurs in the cryptobiotic tardigrades (water bears) and likely in Artemia (brine shrimp). |
||||
Probable Allelotoxicity: |
||||
A purified antibiotic containing chlorine produced by Scytonema hofmanni that inhibits many other cyanobacteria as well as many photosynthetic eukaryotes, (chlorophyceae, euglenophyceae [Euglena] and Rhodophyceae [Porphyridium] were tested) has been named 'cyanobacterin’, C23H23O6Cl. The inhibition is from both intact filaments and crude extract ≥30 µg L-1. Morphological symptoms include disruption of cell walls and loss of cell contents and thylakoids (Mason et al. 1982):
Cyanobacterin is ineffective on non-PS prokaryotes, thus the adaptive advantage is reduction of competition from PS autotrophs. A series of five larger organic extracts called ‘scytophycins’ with cytotoxic and anti-fungal effects were isolated from Scytonema pseudohofmanni (Ishibashi et al. 1986). An additional three scytophycins were later extracted from S. burmanicum (strain DO-4-1) and S. ocellatum (strains DD-8-1 and FF-66-3), as well as ‘tolytoxin’ (Carmeli et al. 1990). The mechanism of inhibition is disruption of microfilament (microtubule) organization. An example of a defense mechanism is induction of tolytoxin by introduction of fungal polysaccharides to a culture of S. ocellatum (Patterson and Bolis 1997). |
||||
Toxicity |
||||
Toxins were known to be produced by Scytonema in the 1990s, as reviewed by Codd (1995). Scytonema cf. crispum, a freshwater strain from New Zealand, produces the neurotoxin saxotoxin (Smith et al. 2011). S. sp. strain BT 23 that grows in soil also produces a heopatotoxic substance not yet identified, that may be novel (Kumaret al. 2000).Desert crusts often contain Scytonema and Nostoc as well as BMAA and microcystin. The toxins may protect the cyanobacteria from oxygen damage by causing chlorosis during nitrogen deprivation, thus reducing photosynthetic rates (Downing et al. 2015). They measured changes in fluorescence of Synechocystis extracts after exposure to various concentrations of microcystin-LR and BMAA. |
||||
Anti-HIV protein: |
||||
‘Scytovirin’ isolated from a culture of S. varium is ‘potently active’ against HIV isolates, targeting HIV envelope glycoproteins (Bokesch et al. 2003). |
||||
Other pharmaceuticals: |
||||
Elastase is a protease that breaks down the elastic fiber elastin in connective tissue, destructive if uninhibited. Elastase inhibitors found in S. hofmanni PCC 7110, scyptolin A and B, have potential use in treating human diseases such as pulmonary emphysema and myocardial damage (Matern et al. 2001). |
||||
Habitat: |
||||
|
On substrates or in dark mats around other algae and vegetation in lakes and on the seacoast. Terrestrial species are found in desert soils. |
||||
References: |
||||
|
Bokesch, H.R., B.R. O’Keefe, T.C. McKee, L.K. Pannell, G.M.L. Patterson, R.S. Gardella, R.C. Sowder II, J. Turpin, K. Watson, R.W. Buckheit Jr. and M.R. Boyd. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 42:2578-2584. Bornet, É. and Flahault, C. (1886 '1887'). Revision des Nostocacées hétérocystées contenues dans les principaux herbiers de France (Troisième fragment). Annales des Sciences Naturelles, Botanique, Septième série 5: 51-129. Carmeli, S., R.E. Moore and G.M.L. Patterson 1990. Tolytoxin and new scytophycins from three species of Scytonema. Journal of Natural Products 53960;1533-1542. Codd, G.A. 1995. Cyanobacterial toxins: Occurrence, properties and biological significance. Water Science Technology 32(4):149-156. Downing, T.G., R.R. Phelan and S. Downing 2015. A potential physiological role for cyanotoxins in cyanobacteria of arid environments. Journal of Arid Environments 112:147-151. Geitler, L. 1932. Cyanophyceae von Europa (1196 pp). In: Rabenhorst, L. (Ed.). Kryptogamen-Flora von Deutschland, Osterreich und der Schweiz. Akademische Verlagsgesellschaft m. b. H. Leipzig. Guiry, M.D. and G.M. Guiry 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 28 February 2013. Ishibashi, M., R.E. Moore and G.M.L. Patterson 1986. Scytophycins, cytotoxic and antimycotic agents from the cyanophyte Scytonema pseudohofmanni. Journal of Organic Chemistry 51:5300-5306. Kumar, A., D.P. Singh, M.B. Tyagi, A. Kumar, E.G. Prasuna and J.K. Thakur 2000. {rpdictopm pf je[atpt4pxom bu the cuampbacvteroi, Scutpme,a s[/ Straom BT 23. Journal of Microbiology Biotechnology 10(3):375-380. Mason, C.P., K.R. Edwards, R.E. Carlson, J. Pignatello, F.K. Gleason and J.M. Wood 1982. Isolation of chlorine-containing antibiotic from the freshwater cyanobacterium Scytonema hofmanni. Science 215 (4531):400-402. Matern, U., L. Oberer, R.A. Falchetto, M. Erhard, W.A. König, M. Herdman and J. Weckesser 2001. Scyptolin A and B, cyclic depsipeptides from axenic cultures of Scytonema hofmanni PCC7110. Phytochemistry 48:1087-1095. Patterson, G.M.L. and C.M. Bolis 1997. Fungal cell-wall polysaccharides elicit an antifungal secondary metabolite (phytoalexin) in the cyanobacterium Scytonema ocellatum. Journal of Phycology 33:54-60. Smith, F.M.J., S.A. Wood, R. van Ginkel, P.A. Broady and S. Gaw 2011. First report of saxitoxin production by a species of the freshwater benthis cyanobacterium, Scytonema Agardh. Toxicon 57:566-573. |
||||
|
Home / Cyanobacteria / Filaments / Pseudo-branches / Scytonema |
||||