|
Home / Chlorophyceae / Unicells / Flagellated / Pyramimonas |
||||
|
|
||||
|
Click on images for larger format |
||||
Name derivation: |
||||
Classification: |
||||
|
Pyramimonas Schmarda, 1849; 40 of 94 species descriptions are currently accepted taxonomically (Guiry and Guiry 2013). Order Pyramimonadales; Family Pyramimonadaceae
|
||||
Morphology: |
||||
|
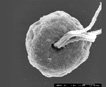
Flagellated Unicells, oval, varying in size by an order of magnitude, 5 – 50 um (O’Kelly 1994), radially symmetric with multiple flagella (4,8,16) arising from an apical pit. In some instances the flagella have scales that vary with species and habitat. If four or eight flagella are present there is one large chloroplast containing a large pyrenoid. In the 16-flagellate species there are two chloroplasts, each with a large pyrenoid. One to two eyespots are common as well as a vacuole in freshwater species. Cell and flagella are covered by organic scales of at least four types (crown, box, underlayer and limuloid), visible only with electron microscopy. Numerous (up to 40) ejectosomes surround the flagellar pit (Zingone et al. 1995). More online (Guiry and Guiry 2013).
|
||||
Similar genera: |
||||
|
|
||||
Scale production |
||||
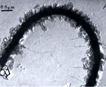
Organic scales that over the cell and flagella originate in cisternae produced by the golgi apparatus. Questions remain about the details of cell liberation to the outside of the cell and flagella (Manton 1966). Many (27) EM micrographs in Manton’s publication present details such as scale alignment, types of scale and scale reservoirs. |
||||
Mixotrophy and Phagotrophy: |
||||
In a somewhat loose definition mixotrophy is the simultaneous ‘mix’ of more than one type of energy acquirement. One type is photosynthesis + phagotrophy.Pyramimonas gelidicola grazed heterotrophic nanoflagellates in permanently ice covered, saline meromictic Ace Lake, Antarctica (68o28.4’ S, 78o11.1’ E), realizing up to 30% of their carbon requirements from bactivory. Maximum phagotrophy was within a pycnocline (density gradient) at 6 – 8 m depth where a chlorophyll maximum containing mainly P. gelidicola occurred (Bell and Laybourn-Parry 2003).Pyramimonas tychotreta maintained in culture in the Antarctic Protist Collection at Woods Hole Oceanographic Institution (WHOI, online) is an obligate autotroph in the sense that it cannot survive for long periods without exposure to light. However it can ingest bacteria (phagotrophy) but only when ambient nutrients are growth limiting, and can do so in either light or dark conditions.. In contrast, P. antarctica continues both photosynthesis and phagotrophy even when nutrient supply is high, again under both light and dark conditions, suggesting that organic carbon as well as other nutrients is augmented. In dark survival experiments original cell concentrations of P. tychotreta were ~1.25 * 105 cells ml-1. (McKie-Krisberg et al. 2014). |
||||
Virus infections: |
||||
|
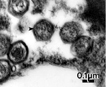
Pyramimonas orientalis cells from Norwegian waters (in the bay by the Marine Biological Field Station of the University of Bergen) are infected by a virus with one of the largest genomes reported, 560 kbp. Lytic cycle lasted 14 – 19 hours, with maximum viroid production at ~30 h. Burst size was ~800-100 viroids per host cell (Sandaa et al. 2001). Pyramimonas tychotreta in sea ice and sea water become infected in relatively small numbers of cells by large (>110 nm capsid diameter) viruses. Extracellular large virus concentration in pack ice was 104 - 106 viroids ml-1 brine. The implication is that blooms of the PS protist are potentially regulated by infection. Only rare protist cells had viroids in their food vacuoles, thus they were not a source of nutrition. (Gowing 2003). |
||||
Predators of Pyramimonas: |
||||
The heterotrophic flagellates Cryothecomonas spp. and an unidentified flagellate were predators in pack ice of the Ross Sea (Ibid.) |
||||
Evolution and Chloroplast genomes: |
||||
The Prasinophyceae include diverse unicellular Chlorophyceae, both flagellated and coccoid. Comparison of cpDNA currently in progress is revising classification of this group and beginning to demonstrate phylogeny of both Chlorophyceae and Streptophytes (green plants). In addition it is now clear that the chloroplasts of Euglenids has been acquired from a prasinophyte similar to Pyramimonas (Turmel et al. 2015). |
||||
Habitat: |
||||
|
Mostly marine in polar seas, with some freshwater species. Psychrophilic protists in the WHOI Antarctic protist culture collection came from the Ross Sea, 65 – 75 south latitude. |
||||
References: |
||||
|
Bell, E.M., and J. Laybourn-Parry 2003. Mixotrophy in the Antarctic phytoflagellate, Pyramimonas gelidicola (Chlorophyta: Prasinophyceae). Journal of Phycology 39:644-649. Gowing, M.M. 2003. Large viruses and infected microeukaryotes in Ross Sea summer pack ice habitats. Guiry, M.D. and G.M. Guiry 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 10 September 2013. Manton, I. 1966. Observations on scale production in Pyramimonas amylifera Conrad. Journal of Cell Science 1:429-438.McKie-Krisberg, A.M., R.J. Gast and R.W. Sanders 2014. Physiological responses of three species of Antarctic mixotrophic phytoflagellates to changes in light and dissolved nutrients. Microbial Ecology. DOI 10.1007/s00248-014-0543-x online.O’Kelly, C.J. 2004. Pyramimonas (online). Protist Image Data (PID).Sandaa, R.-A., M. Heldal, T. Castberg, R. Thyrhaug and G. Bratbak 2001. Isolation and characterization of two viruses with large genome size infecting Chrysochromulina ericina (Prymnsiophyceae) and Pyramimonas orientalis (Prasinophyceae). Virology 290:272-280.Turmel, M., M.-C. Gagnon, C.J. O’Kelly, C. Otis and C. Lemieux 2015. The chloroplast genomes of the green algae Pyramimonas, Monomastix, and Pycnococcus shed new light on the evolutionary history of prasinophytes and the origin of the secondary chloroplasts of euglenids.Zingone, A., J. Throndsen and G. Forlani 1995. Pyramimonas oltmannsii (Prasinophyceae) reinvestigated. Phycologia 34(3):241-249. [online] |
||||