|
Home / Cyanobacteria / Colonies / Aphanocapsa |
||||
|
|
||||
|
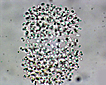
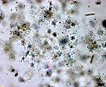
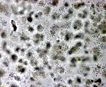
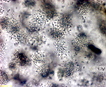
Click on images for larger format |
||||
Name derivation: |
||||
|
"Invisible capsule" (From the Greek, aphanes- hidden or invisible, and kapsa- a capsule) |
||||
Classification: |
||||
|
Aphanocapsa Nägeli 1849; 57 of 105 species descriptions are currently accepted taxonomically (Guiry and Guiry 2013) Order Synechococcales; Family Merismopediaceae |
||||
Morphology: |
||||
|
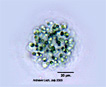
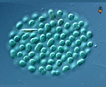
Spherical cells irregularly distributed within mucilage, forming irregularly shaped microscopic or macroscopic colonies . |
||||
Similar genera: |
||||
|
Planktonic forms are often misidentified as Microcystis, which unlike Aphanocapsa, usually forms gas vacuoles. Mucilage surrounding cells of Gleocapsa is denser, each cell being surrounded by individual sheath. Aphanothece cells are more elongate and flatter than Aphanocapsa. |
||||
|
Optimal temperature for growth: |
||||
|
Aphanocapsa 6308 grow best at 35 C (Allen et al. 1980). |
||||
|
Iron deficiency: |
||||
|
Aphanocapsa sp. grows normally in 3 - 36 µM Fe in a mineral medium. At 0.36 µM Fe, severe Fe deficiency with reduced PS and growth occurs, with a reduction in Fe-S trans-membrane electron transport. Photosynthetic production of oxygen was more affected than respiration (Sandmann and Malkin 1983). |
||||
Nitrogen storage: |
||||
Aphanocapsa (strain 6308 that does not fix N) produces N-rich cyanophycin [multi-L-arginyl poly(L-aspartic acid)] granules that were earlier observed in A. cylindrical that is a N-fixer. Granules are most extensive in stationary-phase cells. N-limitation decreased cyanophycin granules. Phosphorus and sulfur limitation, or addition of arginine to the growth medium,as well as low light (~44 µM photons m-2 s-1), all increased the granules. (Allen et al. 1980). |
||||
Habitat: |
||||
|
Planktonic in lakes and ponds, but also benthic on both terrestrial and aquatic surfaces such as plants, rocks and soil. Most species are freshwater, others occur in coastal brackish habitats. Often dominates plankton of relatively pristine lakes, more than 95% in one observed case, Bigsby Lake, located within the Adirondack Park of upstate New York. At least two marine species of Aphanocapsa form a symbiotic relationship with the sponge Ircinia variabilis. In the microhabitat within the sponge, Aphanocapsa produces more phycoerithrin (Sarį 1971) an example of chromatic adaptation. |
||||
References: |
||||
|
Allen, M.M., F. Hutchison and P.J. Weathers. Cyanophycin granule polypeptide formation and degradation in the cyanobacterium Aphanocapsa 6308. Journal of Bacteriology 141(2):687-693. Graham L. E. and L. W. Wilcox. 2000. Algae. Prentice Hall Guiry, M.D. and G.M. Guiry 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 04 September 2013. Jaeger E. C. 1955. A source-book of biological names and terms. Springfield: Charles C Thomas Publisher John D. M., B. A. Whitton
and A. J. Brook, ed. 2008. Nägeli, C. 1849. Gattungen einzelliger Algen, physiologisch und systematisch bearbeitet. Neue Denkschriften der Allg. Schweizerischen Gesellschaft für die Gesammten Naturwissenschaften 10(7): i-viii, 1-139, pls I-VIII. Sandmann, G. and R. Malkin 1983. Iron-Sulfur centers and activities of the photosynthetic electron transport chain in iron-deficient cultures of the blue-green alga Aphanocapsa. Plant Physiology 73:724-728. Sarį, M. 1971. Ultrastructural aspects of the symbiosis between two species of the genus Aphanocapsa (Cyanophyceae) and Ircinia variabilis (Demospongiae). Marine Biology 11:214-221. Wehr J.D. and R. G. Sheath. 2003. Freshwater Algae
of |
||||