|
Home / Cyanobacteria / Colonies / Microcystis |
||||
|
|
||||
|
Click on images for larger format |
||||
Name derivation: |
||||
|
“Small cell” (From the Greek micros –small, and kytos –a hollow vessel, a cell
|
||||
Classification: |
||||
|
Microcystis Kützing 1846; 55 of 139 species descriptions are currently accepted taxonomically (Guiry and Guiry 2013). Order Chroococcales; Family MicrocystaceaeSynonym: Micraloa aeruginosa Kützing 1833 Synonym: Anacystis Meneghini 1837, although recognized as a distinct genus online.Synonym: Chthonoblastus Kützing, 1843 Synonym: Diplocystis (Kützing) Trevisan 1848 Synonym: Chlathrocystis Henfrey 1856 |
||||
Morphology: |
||||
|
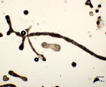
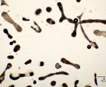
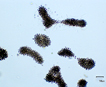
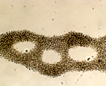
Mainly colonial in nature, unicellular in fast-growing cultures in the absence of flagellate, ciliate, and zooplankton predators. Various species are recognized primarily on various forms of the mucilaginous sheath that varies from watery to viscous, thus changing the shape of the colony. Whether any of these described species is more than a temporary growth form (morphotype or ecad) or is genetically isolated from any other seems unlikely. When treated with various solutions of distilled water with or without various cations including either K+1, Na+1, Mg+2 and Fe+2 alone (but not with Ca+2) the morphotype called Microcystis ichthyoblabe was converted into several different morphotypes that have been described as M. wesenbergii and M. aeruginosa (Li et al. 2013). All three of these morphotypes (‘species’?) were found in successive populations in Lake Taihu, China (Ibid). The same succession had been seen previously in several lakes from China (Jia et al. 2011), Japan (Park et al. 1993, Ozawa et al. 2005) as well as lakes in New Hampshire, USA (see image page) where a variety of different morphotypes have been found at various seasons. Comparisons of ‘morphotypes’ both in the field and in laboratory cultures indicates that they vary sufficiently under different growth conditions that they overlap, and attaching names such as M. aeruginosa, M. ichthyoblabe, M. novacekii, M. viridis or M. wesenbergii is not valid without further evidence (Otsuka et al. 2000). The precautionary tale is that taxonomy based solely on morphology and morphotypes can be misleading when microhabitat and growth history modifies colony shape, confuting not only strains but species and even genera.
|
||||
Molecular sequence: |
||||
Based on partial 16S rDNA sequences, 77 strains of Microcystis have great genotypic homogeneity, in contrast with their conspicuous morphological differences (Lepčre et al. 2000).
|
||||
Nitrogen storage: |
||||
Microcystis uses cyanophycin, multi-L-arginyl-poly(aspartic acid) molecular weight 25,000 – 125,000 daltons (Simon 1971, 1976) to store nitrogen for use when degraded by cyanophycinase when cells are nitrogen starved. The phycobilins also degrade, furnishing available nitrogen during sN-starvation (Boussiba and Richmond 1980).Other potential storage molecules may be the N-rich toxins microcystin and anatoxin.
|
||||
Similar genera: |
||||
|
Various colonial cyanobacteria are easily confused with Microcystis, because of the variability in the “glue” leading to large variability of the colony shape and size. One example is Woronchinia that may be interpreted as a seasonal growth form of Microcystis.
|
||||
Habitat: |
||||
|
Often becomes a dominant plankter in water bodies with excess phosphorus and nitrogen. Microcystis is widespread, present and often dominant in freshwater cyanobacterial blooms, and subject to much analysis and publication because of its toxicity to mammals. Especially cattle and pet dogs have been in the public media after death from drinking lakewater where cyanobacteria blooms have been concentrated in the lee shores of lakes. |
||||
Why dominance: |
||||
|
Reasons why Microcystis become dominant (i.e. outcompete other PS plankton) where soluble reactive phosphorus (SRP) concentration is high (at least 10 µg L-1) include its inedibility that increases with colony size, and possible toxic effect on predators. Pulsed phosphorus (P) loading, as from allochthonous sources (variable runoff from the watershed) or autochthonous (release from heterotrophs) may also positively impact Microcystis. as it may also have adaptive storage of excess P as polyphosphate granules enabling continued growth when SRP declines or is exhausted. An example is nocturnal upward migration of phytoplanktivorous zooplankton that both feed on PS plankton in the epilimnion, and recycle P (and ammonia-N) (Sommer 1985). In addition under low SRP supply it may produce alkaline phosphatase to extract P from dissolved organic forms of P otherwise unavailable. Dreissena polymorpha (‘zebra mussel’) is a nuisance invader in alkaline lakes and streams in North America. Dense mussel populations have changed PS plankton composition, often increasing dominance of Microcystis, as reviewed by Sarnelle et al. (2005). Microcystis (and all cyanobacteria) was a ‘minor component’ of phytoplankton in Lake Erie prior to 1991, with the mussels increasing in abundance since 1988. A large bloom of Microcystis occurred in Summer 1995 thus the mussels cannot control it, and should not be considered ‘ecosystem stabilizers’ (Vanderploeg et al. 2001). In a recent two-year mesocosm experiment, D. polymorpha were placed in large (1.74 * 104 L, i.e. 5 * 104 gallon) tubular enclosures, and caused a decrease in Microcystis under low (~1 µg L-1) SRP but an increase under high (~10 µg L-1) SRP in alkaline Gull Lake, MI USA that had since 2001 been invaded by the mussels and had an abrupt increase in percentage Microcystis from essentially none to 25% of total phytoplankton biomass (Ibid).
|
||||
Known toxins: |
||||
|
microcystin, anatoxin. |
||||
References: |
||||
|
Boussiba, S., and A. Richmond 1980. C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Arch. Microbiol. 125:143-147. Graham L. E. and L. W. Wilcox. 2000. Algae. Prentice Hall Guiry, M.D. and G.M. Guiry 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 30 April 2013. Jaeger, E. C. 1972. A source-book of biological names and terms. 3rd Ed. Charles C. Thomas Publisher Jia, X, D. Shi, M. Shi, R. Li, L. Song, H. Fang et al. 2011. Formation of cyanobacterial blooms in Lake Chaohu and the photosynthesis of dominant species hypothesis. Acta Ecologica Sinica 31:2968-2977. Kützing, F.T. 1843. Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange... Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. pp. [part 1]: [i]-xxxii, [1]-142, [part 2:] 143-458, 1, err.], pls 1-80. Leipzig: F.A. Brockhaus. Lemmermann, E. 1907. Algen I (Schizophyceen, Flagellaten, Peridineen). In: Kryptogamenflora der Mark Brandenburg...3. Band. ( Eds), pp. 1-304. Leipzig. Lepčre, C., A. Wilmotte and B. Meyer 2000. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rDNA sequences. Systematics and Geography of Plants 70:275-283 Li, M., W. Zhu and Q. Sun 2013. Solubilisation of mucilage induces changes in Microcystis colonial morphology. New Zealand Journal of Marine and Freshwater Research. Otsuka, S., S. Suda, R. Li, S. Matsumoto and M.M. Watanabe. 2000. Morphological variability of colonies of Microcystis morphospecies in culture. Journal of General Applied Microbiology 46:39-50. Ozawa, K., H. Fujioka, M. Muranaka, A. Yokoyama, Y. Katagami, T. Homma, et al. 2005. Spatial distribution and temporal variation of Microcystis species composition aned microcystin concentration in Lake Biwa. Environmental Toxicology 20:270-276. Park, H.D., M.F. Watanabe, K. Harada, M. Suzuki, H. Hayashi, and T. Okino 1993. Seasonal variations of Microcystis species and toxic heptapeptide microcystins in Lake Suwa. Environmental Toxicology and Water Quality 8:425-4325. Sarnelle, O., A.E. Wilson, S.K. Hamilton, L.B. Knoll and D.F. Raikow 2005. Complex interactions between the zebra mussel, Dreissena polymorpha, and the harmful phytoplankter, Microcystis aeruginosa. Limnology and Ocenanography 50(3):896-904. Simon, R.D. 1971. Cyanophycin granules from the blue-green alga Anabaena cylindrical: a reserve material consisting of co-polymers of aspartic acid and arginine. Proceedings of the National Academy of Science U.S.A. 68:265-267. Simon, R.D. 1976. The biosynthesis of multi-L-Arginyl-poly(l-aspartic acid) in the filamentous cyanobacterium Anabaena cylindrical. Biochimica et Biophysica Acta 422:407-418. Sommer, U. 1985. Comparison between steady state and non-steady state competition: Experiments with natural phytoplankton. Limnology and Oceanography 30(2):335-346. Vanderploeg, H.A., J.R. Liebig, W.W. Carmichael, M.A. Agy, T.H. Johengen, G.L. Fahnenstiel, and T.F. Nalepa 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Scinces 58:1208-1221. Wehr J.D. and R. G. Sheath. 2003. Freshwater Algae of North America. Academic Press (Imprint of Elsevier) |
||||