|
|
|
|
||
|
Click on images for larger format |
||||
Name derivation: |
||||
|
‘Wooly monad’ (dressed in ‘wool’) because of abundance of silicaceous bristles in some species. |
||||
Classification: |
||||
|
Mallomonas Perty 1852; 179 of 300 species descriptions are currently accepted taxonomically (Guiry and Guiry 2014). Order Synurales; Family Mallomonadaceae |
||||
Morphology: |
||||
|
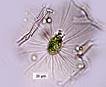
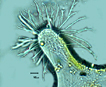
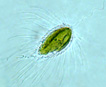
Biflagellate unicells covered with silicaceous scales and articulating bristles that lie flat streamlining the cell during forward motion, and lie perpendicular to cell axis when at rest. Heterokonts – one flagellum bears mastigonemes, the other does not. Cells are globose to elongate. Scales and bristles are produced within separate cisternae of the golgi apparatus (Wujek and Kristiansen 1978) from an organic template. In many cases species are defined by the morphology of both scales and bristles. A description with SEM photographs with a discussion of some fossil Eocene samples and modern specimens is an excellent example of species taxonomy (Siver et al. 2009. See also several additional related publications with new species descriptions by Peter Siver (Ibid.) Scales are ornate and include a central holster into which the bristles (that develop in separate cisternae) are inserted extracellularly. Bristles vary in morphology, many with a hooked proximal end (‘foot) and a ‘helmet’ or saw-toothed distal end. The mechanism by which scales and bristles are moved to the outside of the plasma membrane has been theorized to occur in different manners and to date has not been fully verified. In one case the bristles near the cell posterior were observed via video recording to be extruded proximal end first, then with manipulation by a ‘fibrillar complex’ associated with the plasma membrane, rotated 180o outside the cell. Then the bristle base was subsequently drawn back into the cell and hooked into one of the scales (Beech et al. 1990). In the case of bristles near the anterior of the cell, they were extruded distal end (tip) first. In both anterior and posterior cases the bristles were extruded after and underneath the previously formed scale armor, accompanied by a ‘cellular protuberance (Ibid.). Extracellular manipulation seems almost metaphysical! In another the scales (in Synura) were thought to be aligned into rows while still within the cell, then exported as a unit to form the outer cell wall (Leadbeater 1986). A more plausible explanation is that individual scales are exported one at a time, and in Mallomonas the separately-produced bristle follows the scale across the cell membrane, extruded distal end first, hooking into the holster of the scale as they proceed to form a unit of the outer siliceous wall (Siver and Glew 1990). Whatever the precise mechanism of outer silicaceous wall formation, Siver and Glew (1990) stress that the released scales and bristles would be potentially lost were it not for production by the cell of an adhesive material previously predicted (McGrory 1976). Apparently the number of scales per cell is species specific (as many as 150 bristles has been observed), and their production stops when the cell is mature. Further growth could be accommodated by expansion of the siliceous wall such that the overlap between scales is reduced (Siver and Glew 1990). During asexual reproduction the outer layer of bristles and scales splits while karyogamy occurs with longitudinal division of the cell. The dividing cell may also ‘back out’ of the outer wall while dividing. The two daughter cells develop new scales and bristles. During sexual reproduction isogametes develop. After fusion the zygotes develop silicified walls. Stomatocysts have been observed in some species, occasionally with a collar around the stoma. For additional morphological detail see Guiry and Guiry (2014). |
||||
Similar genera: |
||||
|
|
||||
Habitat: |
||||
|
Mostly freshwater but a few rare marine species. Global distribution. The presence of Mallomonas is a key sign of polluted waters and shows the importance of phytoplankton composition in bodies of water. Mallomonas is a good indicator of deteriorating conditions in water (Wan Maxnah, 2014). |
||||
References: |
||||
|
Beech, P.L., R.L. Wetherbee, and J.D. Pickett-Heaps 1990. Secretion and deployment of bristles in Mallomonas splendens. Journal of Phycology 26:112-122. Guiry, M.D. & Guiry, G.M. 2012. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org Leadbeater, B.S.C. 19896. Scale-case construction in Synura petersenii Korsh. (Chrysophyceae). In: Chrysophytes: aspects and problems. J. Kristiansen and R.A. Andersen (Eds.). Cambridge University Press, Cambridge. pp. 121-131. McGrory, C.B. 1976. A non-siliceous component of chrysophyte scales. British Phycology Journal 11:197. Perty, M. 1852. Zur Kenntniss kleinster Lebensformen: nach Bau, Funktionen, Systematik, mit Specialverzeichniss der in der Schweiz beobachteten. pp. 1-228, pls I-XVII. Bern: Verlag von Jent and Reinert. Siver, P.A., and J.R. Glew 1990. The arrangement of scale and bristles on Mallomonas (Chrysophyceae): a proposed mechanism for the formation of the cell covering. Canadian Journal of Botany 68:374-380. Siver, P.A., A.M. Lott and A.P. Wolfe 2009. Taxonomic significance of asymmetrical helmet and lance bristles in the genus Mallomonas (Synurophyceae) and their discovery in Eocene lake sedijments. European Journal of Phycology 44(4):447-460. Wan Maznah W.O. and A. Makhlough 2014. Water quality of tropical reservoir based on spatio- temporal variation in phytoplankton composition and physico-chemical analysis. Islamic Azad University. Wujek, D.E. and J. Kristiansen 1978. Observations on bristle- and scale-production in Mallomonas caudata (Chrysophyceae). Arch. Protistenkd. 120:213-221. |
||||
|
Home / Synurophyceae / Mallomonas |
||||