|
Home / Cyanobacteria / Colonies / Nostoc or return to: |
||||
|
|
||||
|
|
||||
|
|
|
|||
|
Click on images for larger format |
||||
Name derivation: |
||||
|
Name believed to have been invented by a 15th century Swiss-German scientist, philosopher, astrologer and alchemist, considered the 'founder of modern toxicology' (Borzelleca 2000) named Auroleus Phillipus Theophrastus Bombastus von Hohenheim (Paracelsus), 1493-1541, considered one of the first systematic botanists, a genius, a heavy drinker with his students and a woman chaser (Potts 1997). Hohenheim called terrestrial colonies ‘Nostoch’, combining Nosthryl = nostril (Old English) and Nasenloch = nostril (German). Due to the hydration and sudden appearance in the summer of the colonies, Paracelsus postulated that they fell from the sky as ‘excrement blown from the nostrils of some rheumatick planet’ (Ibid.). More about Hohenheim online. |
||||
Classification: |
||||
|
Nostoc Vaucher ex Bornet and Flahault 1886; 68 of 346 species descriptions are currently accepted taxonomically (Guiry and Guiry 2013). Order Nostocales; Family Nostocaceae Synonym in PhycoKey - Nostochopsis Wood ex Bornet and Flahault, 1886 (tropical) In PhycoKey you can find Nostoc either as a colony or a filament because it is a colony of filaments. |
||||
Morphology: |
||||
|
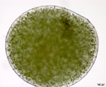
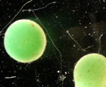
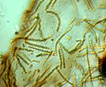
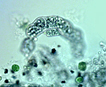
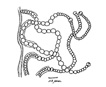
Spherical colonies of twisted trichomes enveloped within a firm gelatinous matrix that can be yellow, brown or black; a variety of colors and sizes can be seen in the same habitat, begging the question of why coloration varies. Colonies of Nostoc pruniforme can reach large sizes of up to 22 cm in diameter (Dodds and Castenholz 1988). Macroscopically as colonies they are readily identifiable and uniquely Nostoc. Microscopically they could be confused with Anabaena unless the enclosing sheath is also observed. The surface of colonies has a ‘skin’ more viscous than the internal gel. Some colonies appear to have a hollow center, with trichomes embedded in peripheral gel. Color of the gel matrix is highly variable even within the same population and habitat. The youngest (1 – 2 mm diameter) colonies are cyan to emerald. We have collected older lightly pigmented turquoise, emerald and golden colonies and at the same time dark nearly black colonies from 1 – 6 mm diameter. Apparently the gel color is due in large part to the water-soluble phycobilins, especially phycoerithrin (Mollenhaur et al. 2000). Outer thallus teguments are sometimes covered by calcareous deposits (Ibid.).
|
||||
Similar genera: |
||||
|
Anabaena also forms colonies of filaments within a mucilaginous matrix, but filaments are less coiled than in Nostoc. Nostoc typically forms akinetes halfway between heterocysts, while Anabaena typically forms akinetes from cells adjacent to heterocysts.
|
||||
Reproduction: |
||||
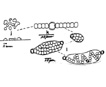
In an undisturbed aquarium filled with a weak ASM1 growth medium we have seen eruptions from colonies recently collected from a lake (Goose Pond, Shapleigh ME USA). The eruptions contain hundreds of trichomes and free heterocysts. We have speculated that the weak ion concentration may be hypotonic, driving a flux into the colonies, causing the ‘skin’ to rupture and the trichomes to erupt.Individual trichomes with intercalary heterocysts located in the center, with essentially equal numbers of vegetative cells on each side, break in half (fractionation), forming two independent shorter trichomes without heterocysts, at the same time freeing their heterocyst that no longer can be functional in N-fixation. Within hours the trichomes now form terminal heterocysts at each end, and continue vegetative cell division and growth.Within two days a film develops on the water surface (neuston) containing trichomes released from colonies, and free heterocysts. The trichomes proliferate, initiating new colonies of a few trichomes during the next two days. Microcolonies growing at 25 C have been produced within 15 days (Deng et al. 2008).
|
||||
Photosynthetic activity: |
||||
|
Photosynthesis
per unit chlorophyll was highest in the outer layer and least in the inner
layer (geometric center of Nostoc
sphaeroides colonies. In contrast
chlorophyll, phycoerithrin and phycocyanin were
highest in the inner layer of 3 cm colonies (Deng et al. 2008). Similarly, rates of both photosynthesis and
respiration in N. pruniforme are
highest in a layer of high density of filaments near the colony margin to the
center in
colonies with a diameter from 1 to 10 cm (Arvidson
and Baker 2017). The cycling of dissolved oxygen inside the colonies as measured with an optical (fluorescence quenching) microprobe is relatively extreme, varying from 4x relative saturation within two hours of exposure to white light, and dropping to anoxia again within two hours of darkness. The rate of both net photosynthesis (PSn) and R increased to a maximum within ~ 30 minutes in light and dark respectively, then dropping to no net change after two hours. One interpretation of the rapid increase and decrease in both PSn is a rapid increase in photorespiration. An explanation for the rise and fall in R during dark exposures is the rapid consumption of photosynthates generated in the previous light exposure, followed by photosynthate depletion and negligible respiration (Ibid.).
|
||||
Age of colonies: |
||||
Although formation of microcolonies (50 – 200 µm diameter) occurs rapidly (15 days), the age of large macrocolonies remains a mystery, with estimates from months to centuries, even millennia for the largest (>20 cm diameter) and oldest. Adding to the mystery is the persistence of the gel exudate as static colorless colonies lacking cells and pigments. When did they become ghosts, and how long will they remain intact?One underwater photographer has seen benthic colonies of Nostoc pruniforme only during late summer (September-October) in lakes in Maine USA, and apparent rapid growth in his aquarium (D. Roberge, personal communication). Is it possible that the colonies remain unobserved when growing under a canopy of macrophytes? Can they also become buried during much of the time, and re-emerge in late summer by increasing their buoyancy?At the opposite end of the growth rate spectrum Nostoc pruniforme is considered to be the slowest growing of all cyanobacteria, and growth rate decreases with age (Dodds and Castenholz 1988). Many reasons for the extremely slow growth rate reviewed by Sand-Jensen (2014) include the potential for cryptobiology – prolonged periods of dessication and/or freezing in terrestrial habitats; slow diffusion rates into and within the matrix of gel colonies; low external nutrient concentrations including ions of inorganic carbon, nitrogen and phosphorus; low ambient light in the benthos of lakes; short ice-free periods in subarctic and arctic lakes; low temperature (0-4 C); high absorbance of light within the colony by both cells and pigments secreted into the gel.Even when growing in the benthos of eutrophic lakes, the light is limited by high absorbance through the water column, both in the metalimnnion and the epilimnion during (mostly) cyanobacterial blooms.Sand-Jensen suggests with mathematical models that low diffusion rates may be the major controller of growth rate. Small changes in water flow make large differences in diffusion.Doubling time in young gel colonies has been measured from 2 weeks (N. pruniforme, N. commune) to 2.4 years for N. zetterstedtii with an optimal temperature of ~ 25 C. Rates of both photosynthesis and respiration increased with temperature in the range 6 – 25 C (Møller et al. 2014).Epiphytes on the colony surface retard growth. The snail Vorticifex effuse apparently grazed epiphytes in Mare’s Egg Spring, Oregon and this had a positive effect on growth (Dodds and Castenholz 1987).
|
||||
Stealth – erratic seasonal visibility: |
||||
I suspect that reports of rapid growth and short seasonal appearance are affected by the ability of Nostoc pruniforme to disappear and reappear. Because it’s habitat is sedentary on sandy or muddy lake bottoms, and because it is nearly neutrally buoyant, it can be moved around by water currents, including those created during vernal or autumnal mixis of the entire water column. In addition, we’ve collected soft sediments and found buried colonies up to 5 cm diameter and as deep as ~10 cm. Further, we’ve collected several colonies by sweeping a long-handled net through benthic macrophytes (e.g. Myriophyllum sp.) where the colonies were invisible from the surface.In cases where the colonies are buried at least seasonally (winter/spring in temperate lakes) they may somehow emerge either by water currents or possibly increasing their buoyancy slightly – even though gas vesicles may not be produced.A related problem we share with Mollenhauer et al. (2000) is the problem of confusion by those unfamiliar with identification to report Nostoc when it is instead the low-viscosity gel colonies of the ciliate Ophrydium versatile colored green with its enbiont Zoochlorella, and forming colonies several centimeters in diameter. |
||||
Pharmaceuticals: |
||||
Extracts with cytotoxic effects can be useful anticarcinogens if they are effective against cancer cells, such as a strain of Nostoc (BECID19 from the Baltic Sea (Gulf of Finland) and several Anabaena and Nodularia strains (Surakka et al. 2005). |
||||
Toxins: |
||||
Degree of cytoxicity of 82 terrestrial Nostoc strains varied between habitats, with the lowest percentage of strains from tropical and desert zones, more in polar regions, still more from temperate continental zones, and the most in symbiotic strains (Hrouzek et al. 2009).
|
||||
Habitat: |
||||
|
Free-living colonies occupy a great diversity of habitats including freshwater, brackish, marine, terrestrial (damp surfaces). Nostoc pruniforme is benthic in ponds, lakes, and pools. Growth is faster in alkaline ‘hardwater’ than in slightly acidic ‘softwater’ lakes. Some are symbiotic, growing inside fungi, mosses, ferns, and coralline above-ground roots of cycads. Terrestrial Nostoc colonies
are considered an unsightly and slippery nuisance in the UK where they often
grow on damp walkways between stones.
However, with its capacity for N-fixation to ammonia, Nostoc is an
important fertilizer of rice paddies. Nostochopsis is similar or same genus limited to tropical zone, rarely subtemperate.
|
||||
References: |
||||
|
Arvidson, B., and A.L. Baker 2017. Changes in dissolved oxygen concentration
within colonies of Nostoc pruniforme
measured with
fluorescence quenching microbe.
UNH Center for Freshwater Biology Research.
Borzelleca, J.F. 2000. Profiles in toxicology. Paracelsus: Herald of Modern Toxicology. Toxicological Sciences 53:2-4. Deng, Z., Q. Hu, F. Lu, G. Liu and Z. Hu 2008. Colony development and physiological characterization of the edible blue-green alga, Nostoc sphaeroides (Nostocaceaen, Cyanophyta). Progress in Natural Science 18:1475-1483. Dodds, W. K. and R. W. Castenholz 1987. Efects of grazing and light on the growth of Nostoc pruniforme. British Journal of Phycology 33:219-227. Graham, L., and L. Wilcox 2000. Algae. Prentice Hall Guiry, M.D. and G.M. Guiry 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 04 September 2013. Hrouzek, P., P. Tomek, A. LukeSová, J. Urgan, L. Voloshko, B. Pushparaj, S. Ventura, J. LukavskY, D. Stys and J. KopeckY 2009. Cytotoxicity and secondary metabolites production in terrestrial Nostoc strains, originating from different climatic/geographic regions and habitats: Is their cytotoxicity environmentally dependent? Environmental Toxicology 26(4):345-358. Jaeger E. 1972. A source-book of biological names and terms. 3rd Ed. Charles C. Thomas Publisher Mollenhauer, D., R. Bengtsson, E-A Lindstrom and M. Oslash 2000. Macroscopic cyanobacteria of the genus Nostoc: a neglected and endangered constituent of European inland aquatic biodiversity. European Journal of Phycology 34:349-360. Møller, C.L., M.T. Vangsøe and K. Sand-Jensen 2014. Comparative growth and metabolism of gelatinous colonies of three cyanobacteria, Nostoc commune, Nostoc pruniforme, and Nostoc zetterstedtii, at different temperatures. Potts, M. 1997. Etymology of the genus name Nostoc (Cyanobacteria). International Journal of Systematic Bacteriology 47(2):584. Sand-Jensen, K. 2014. Ecophysiology of gelatinous Nostoc colonies: unprecedented slow growth and survival in resource-poor and harsh environments. Annals of Botany 114:17-33. Surakka, A., L.M. Sihvonen, J.M. Lehtimäki, M. Wahlsten, P. Vuorela and K. Sivonen 2005. Benthic cyanobacteria from the Baltic Sea contain cytotoxic Anabaena, Nodularia, and Nostoc strains and an apoptosis-inducing Phormidium strain. Environmental Toxicology 20:285-295. Wehr J., and R. Sheath 2003. Freshwater Algae of North America. Academic Press (Imprint of Elsevier) Whitford L., and G. Schumacher 1973. A manual of fresh-water algae. Sparks Press |
||||
|
Home / Cyanobacteria / Colonies / Nostoc or return to: |
||||
| / Filaments / Unbranched / Untapered / Heterocysts / Visible Sheath | ||||